Pipeline
ABL001
HomePipelineABL001
Publications
Lee D et al. Simultaneous blockade of VEGF and Dll4 by HD105, a bispecific antibody, inhibits tumor progression and angiogenesis. MAbs. 2016 Jul;8(5):892-904.
Choi WH et al. Development, validation, and application of ELISA for detection of anti-HD105 antibodies in pre-clinical safety evaluation using monkeys. J Pharm Biomed Anal. 2016 Nov 30;131:309-315.
Lee J et al. Phase 1a study results investigating the safety and preliminary efficacy of ABL001 (NOV1501), a bispecific antibody targeting VEGF and DLL4 in metastatic gastrointestinal (GI) cancer. ASCO 2019
You WK et al. Summary of Phase 1a Dose Escalation Clinical Study Data for Dual Angiogenic Bispecific Antibody Targeting VEGF and DLL4 (ABL001/NOV1501/TR009) in Patients with Previously Treated Solid Tumors. Peptalk 2020
Dual VEGF/DLL4 Inhibition Overcomes VEGF Therapy Resistance
Dual Blockade of VEGF & DLL4 overcomes VEGF resistance
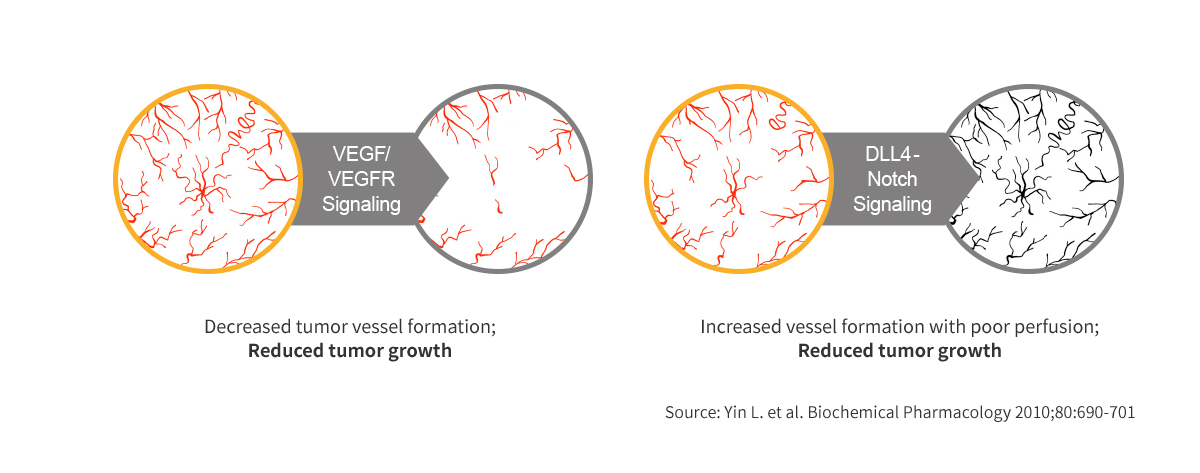
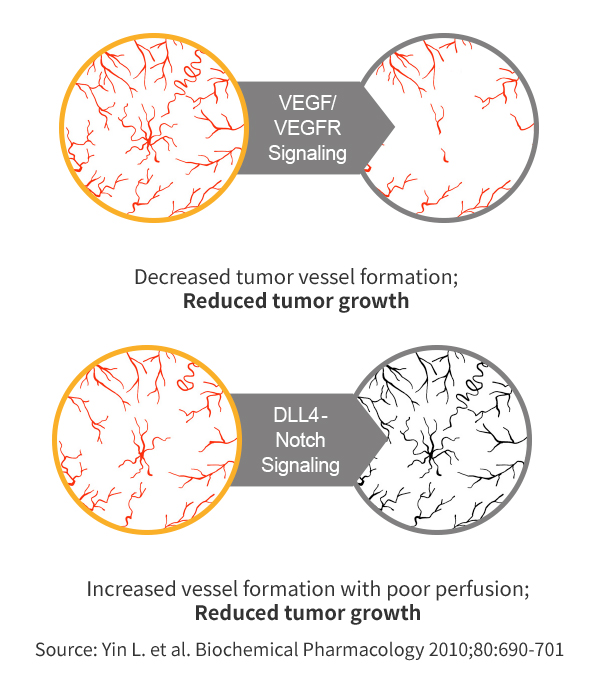
- VEGF/DLL 4
- Drastic reduction in size of tumors compared to Anti-DLL4 and Anti-VEGF
ABL001 is BETTER than Avastin
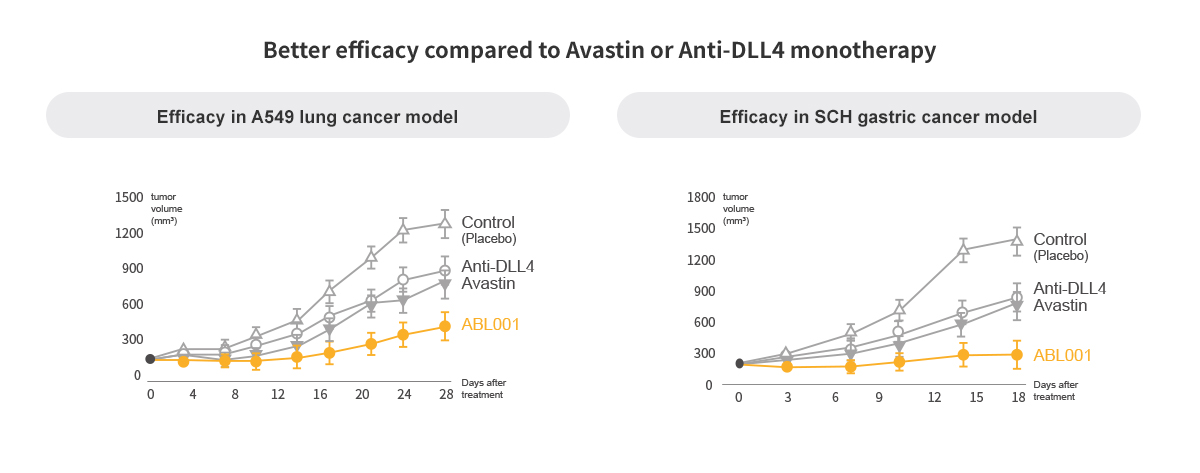
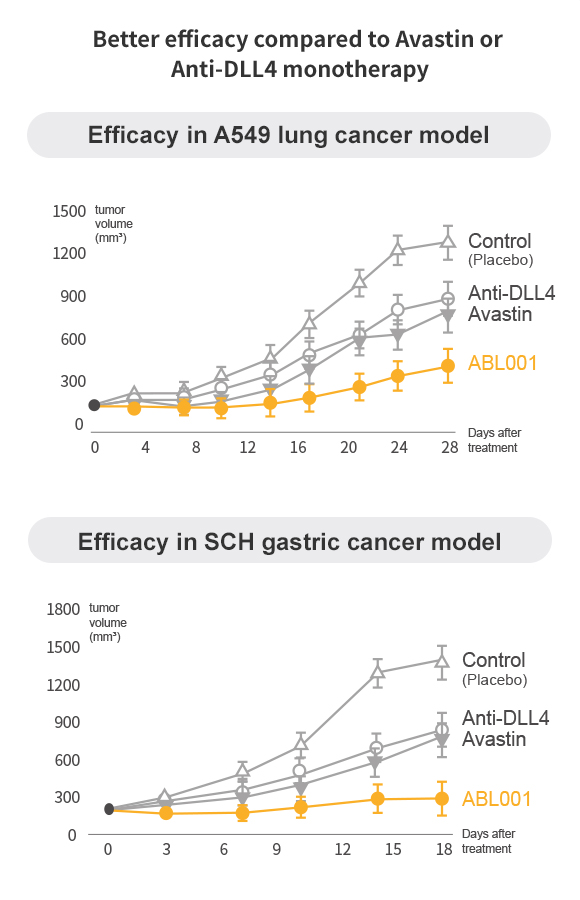
Potential Success of ABL001
ABL001 : First BsAb Clinical Trial in Korea
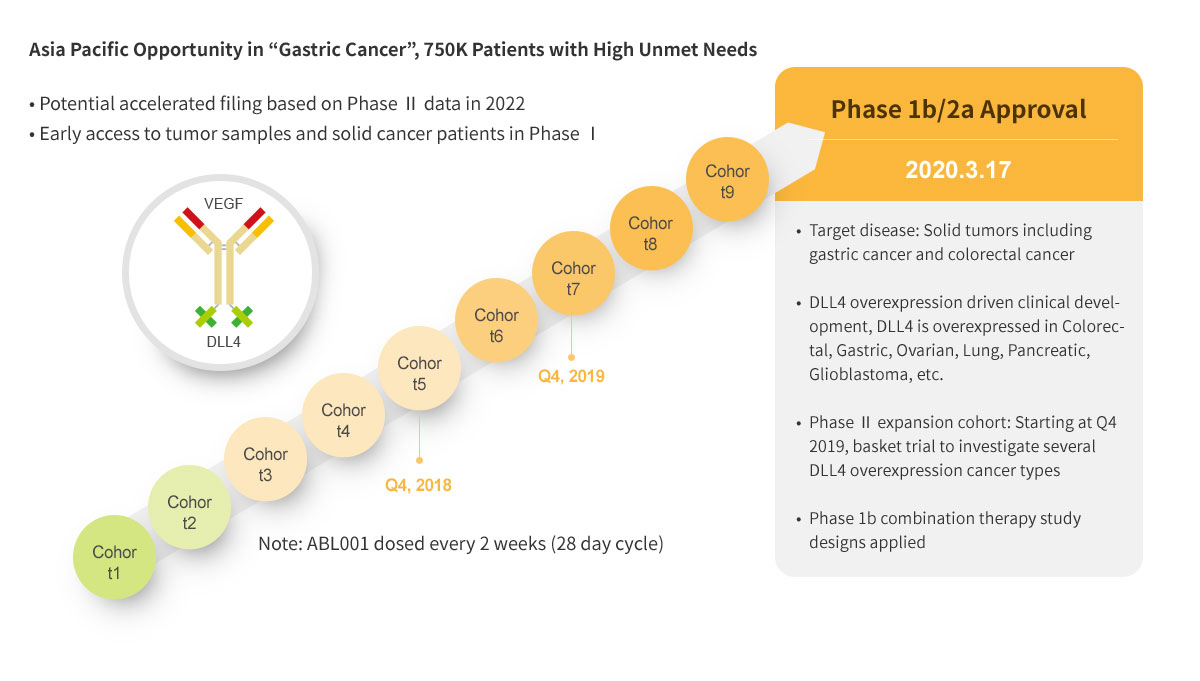

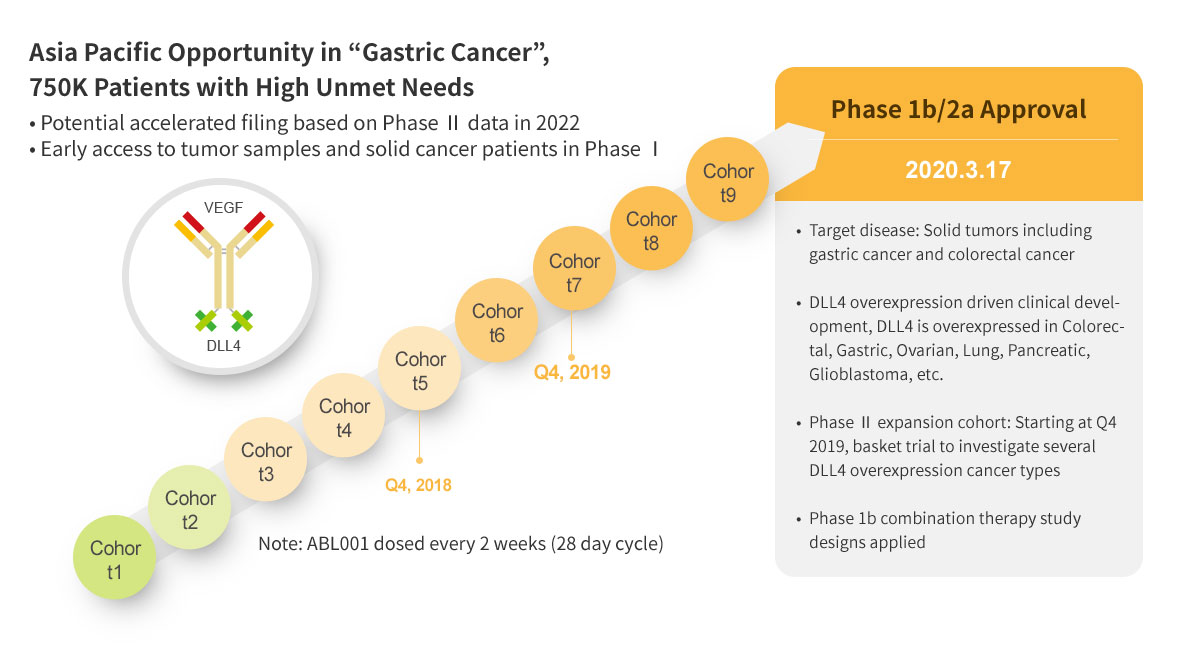
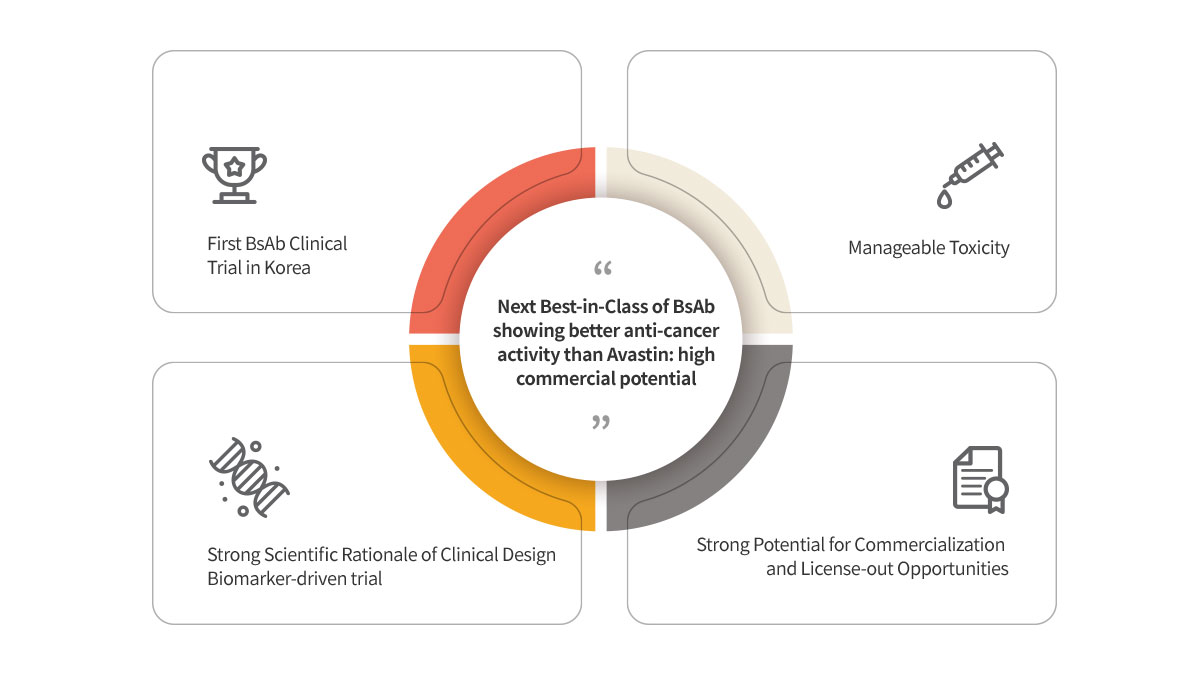
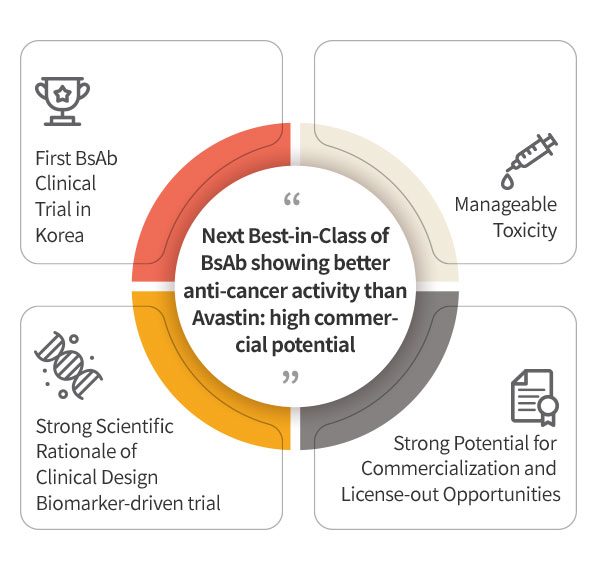
ABL001 : NEXT BEST-IN-CLASS BISPECIFIC ANTIBODY