Pipeline
ABL001
HomePipelineABL001
-
- Pipeline
- ABL001
-
- Program Target
- VEGF x DLL4
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1a/1b)
- Summary
- Better anti-cancer efficacy of ABL001 at in vitro/in vivo studies are confirmed with cancer patients in clinical trial (phase 1a/1b).
ABL001 can be developed as the next generation of anti-angiogenic therapy.
MOA of ABL001
Dual blockade of VEGF & DLL4 overcomes VEGF resistance
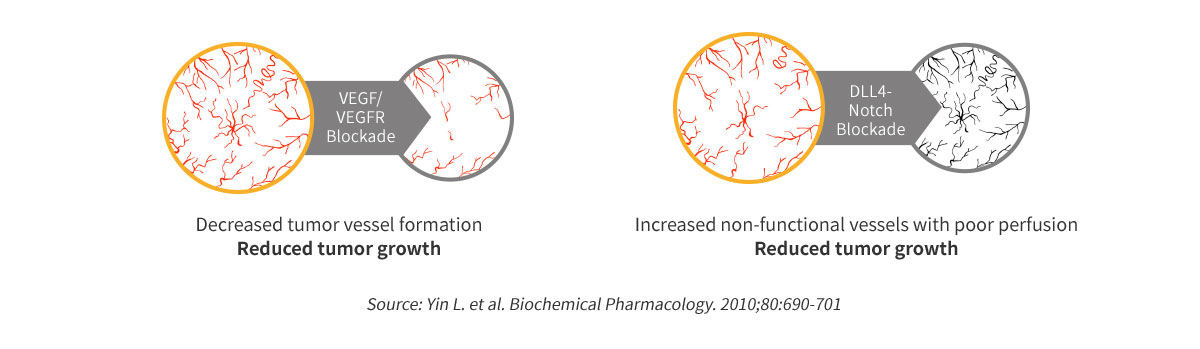
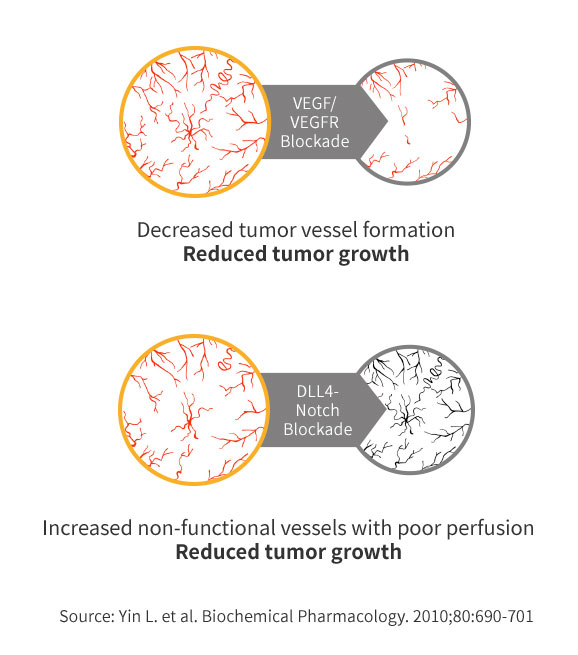
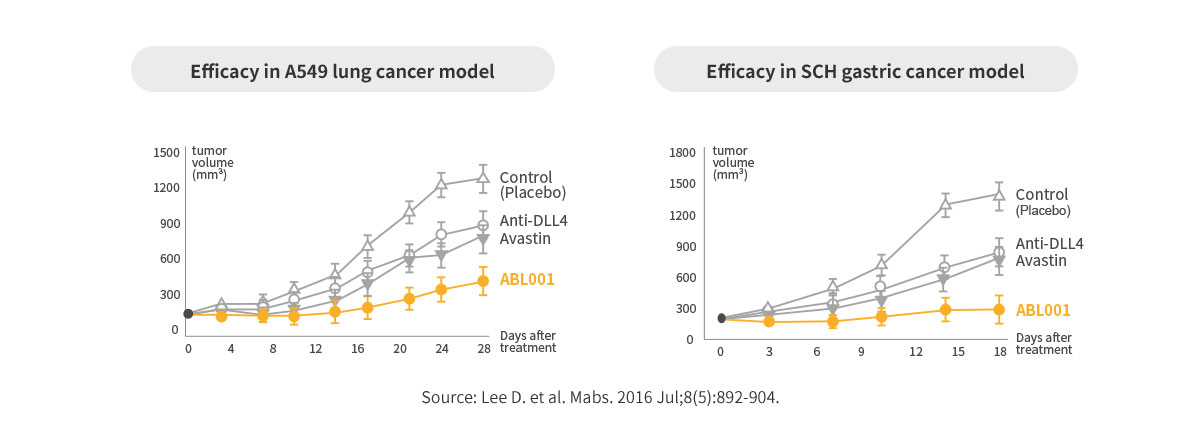
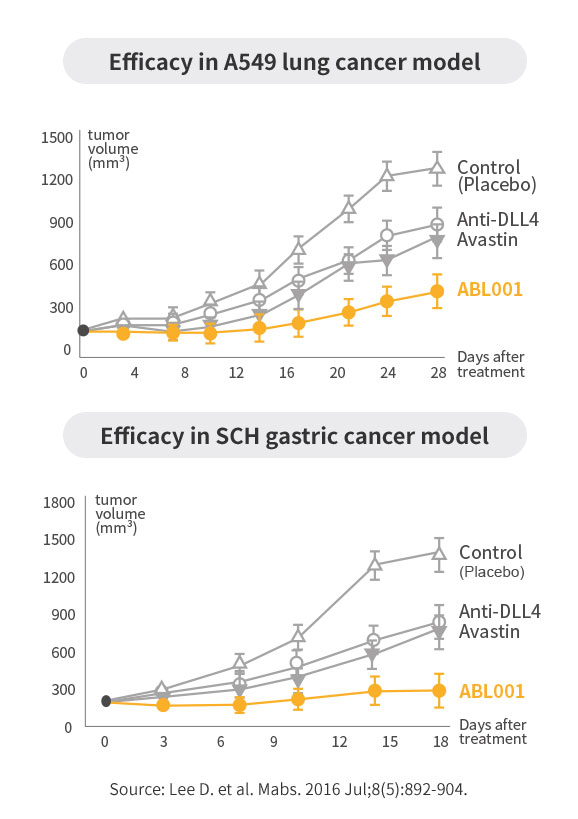
POC of ABL001 in Preclinical Study
Better efficacy compared to Avastin or anti-DLL4 monotherapy
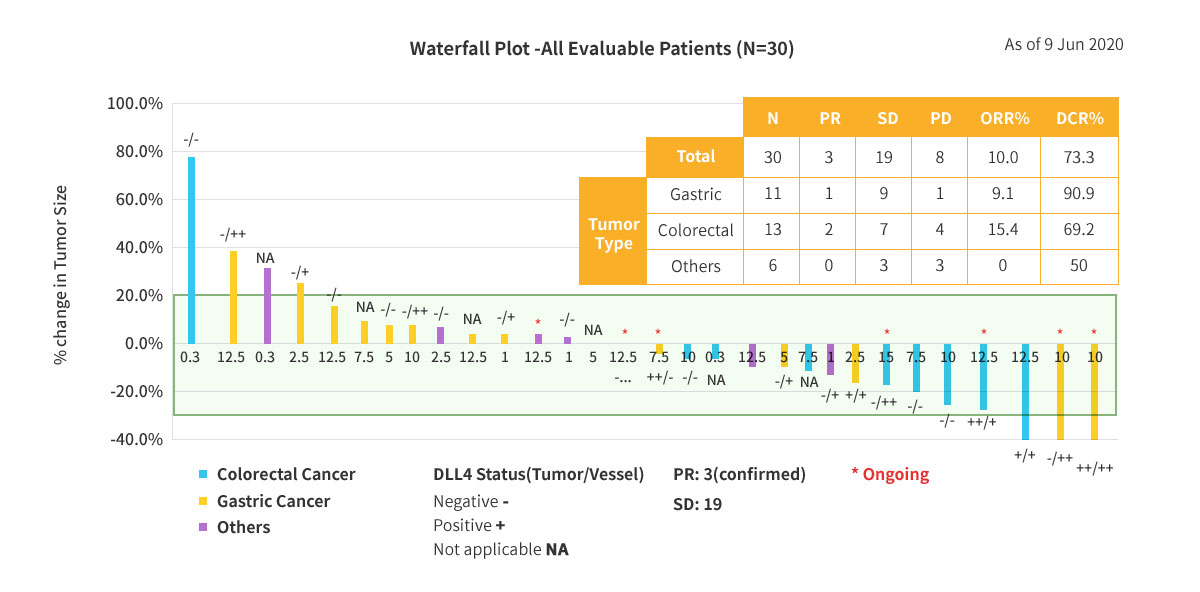
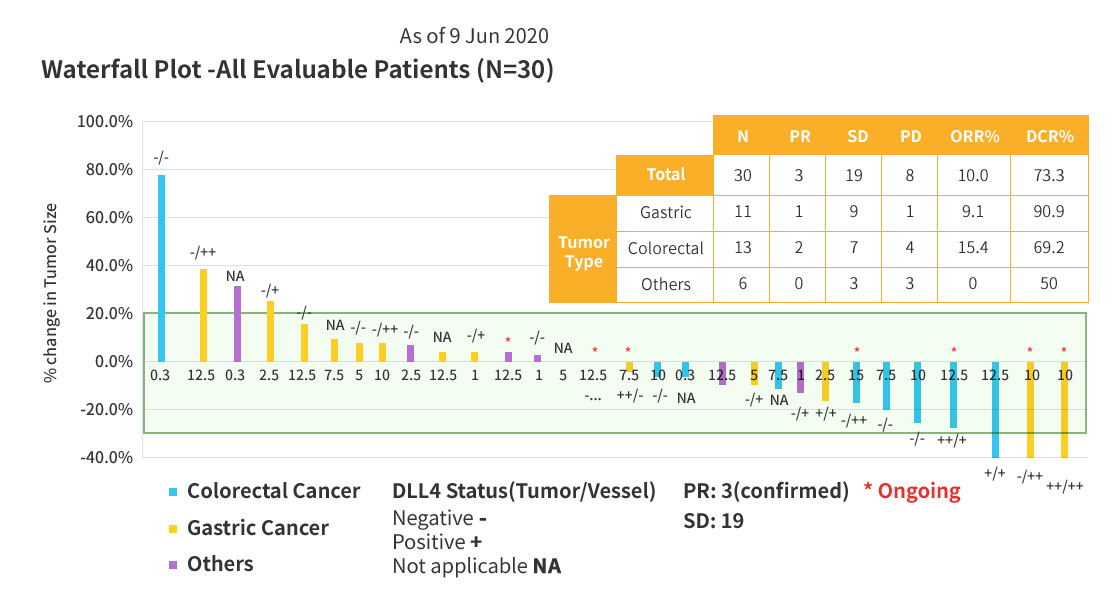
Clinical Response of ABL001
3 Patients showed confirmed partial response (PR) in phase 1a/1b study

Publications

Posters


