Pipeline
ABL503
HomePipelineABL503
-
- Pipeline
- ABL503 (Ragistomig)
-
- Program Target
- PD-L1 x 4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
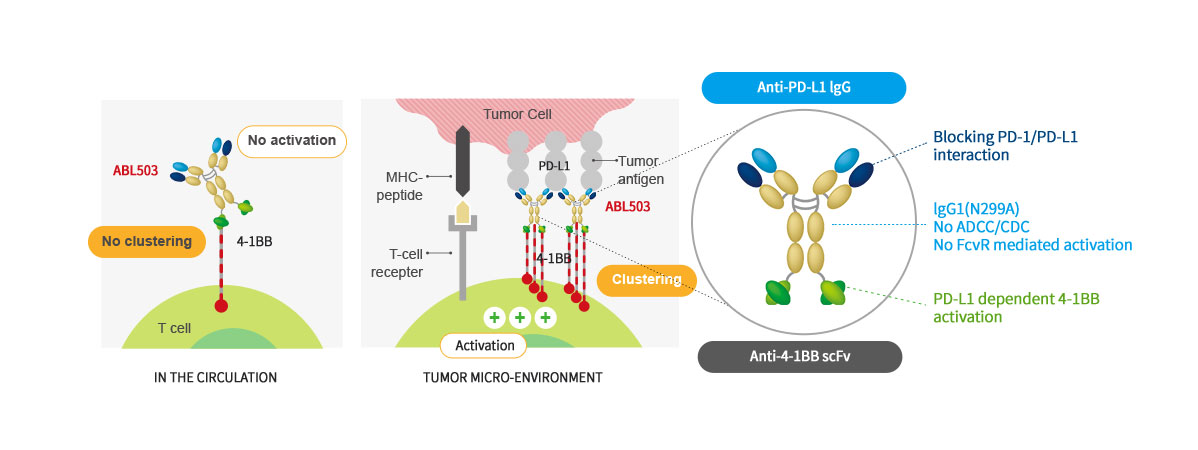
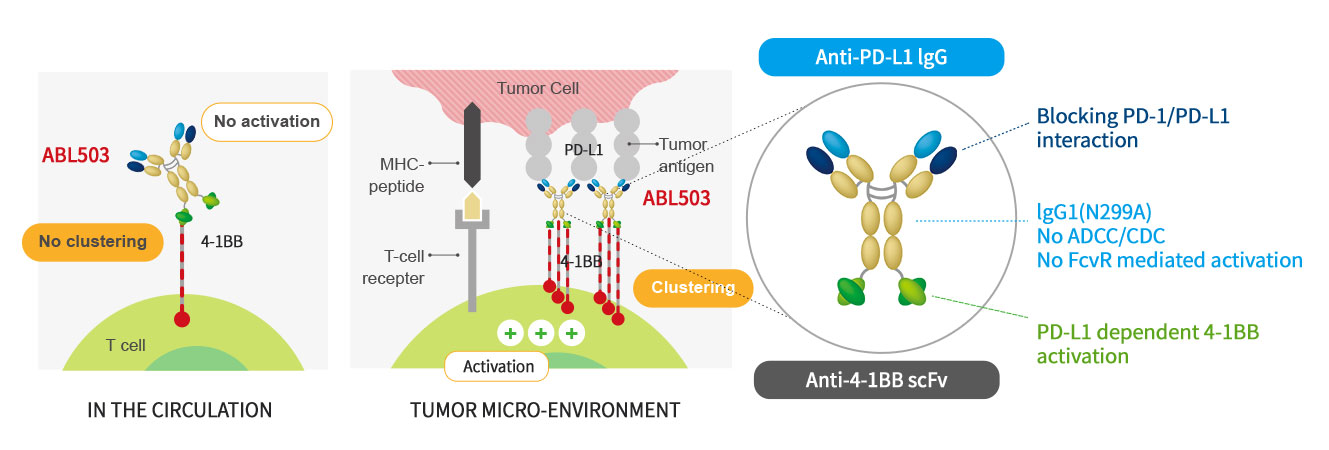
- ABL503 is a bispecific antibody combining PD-L1 checkpoint pathway with 4-1BB agonistic activity to overcome the current limitation of PD-(L)1 therapy and 4-1BB related toxicity. ABL503 is full length anti-PD-L1 mAb (Fc-silenced human IgG1) fused with scFv of anti-4-1BB engaging mAb. ABL503 possesses unique properties that 4-1BB activation signal is only induced in the presence of PD-L1 expressing tumors. ABL503 shows superior anti-tumor activity in an animal model system than PD-L1 alone, 4-1BB alone or combination of both with immunological memory resistant to rechallenge with same tumors.
Structure and Mechanism of Action

Encouraging anti-tumor activity at potential efficacious dose level (3 mg/kg and 5 mg/kg)
All responses were observed at the potential efficacious dose level. (7 out of 26 efficacy-evaluable patients at 3 and 5 mg/kg): 1 complete response (CR) and 6 partial responses (PRs)
Best percent change from baseline in tumor size
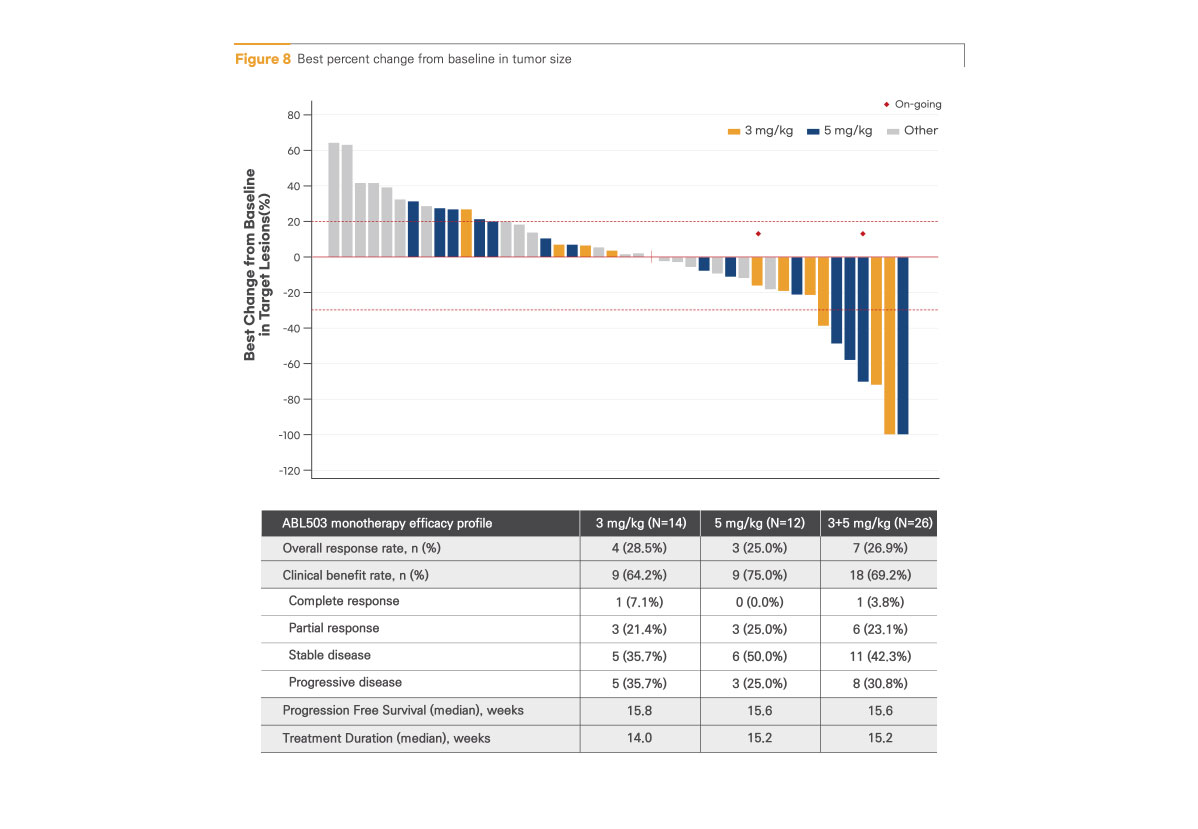
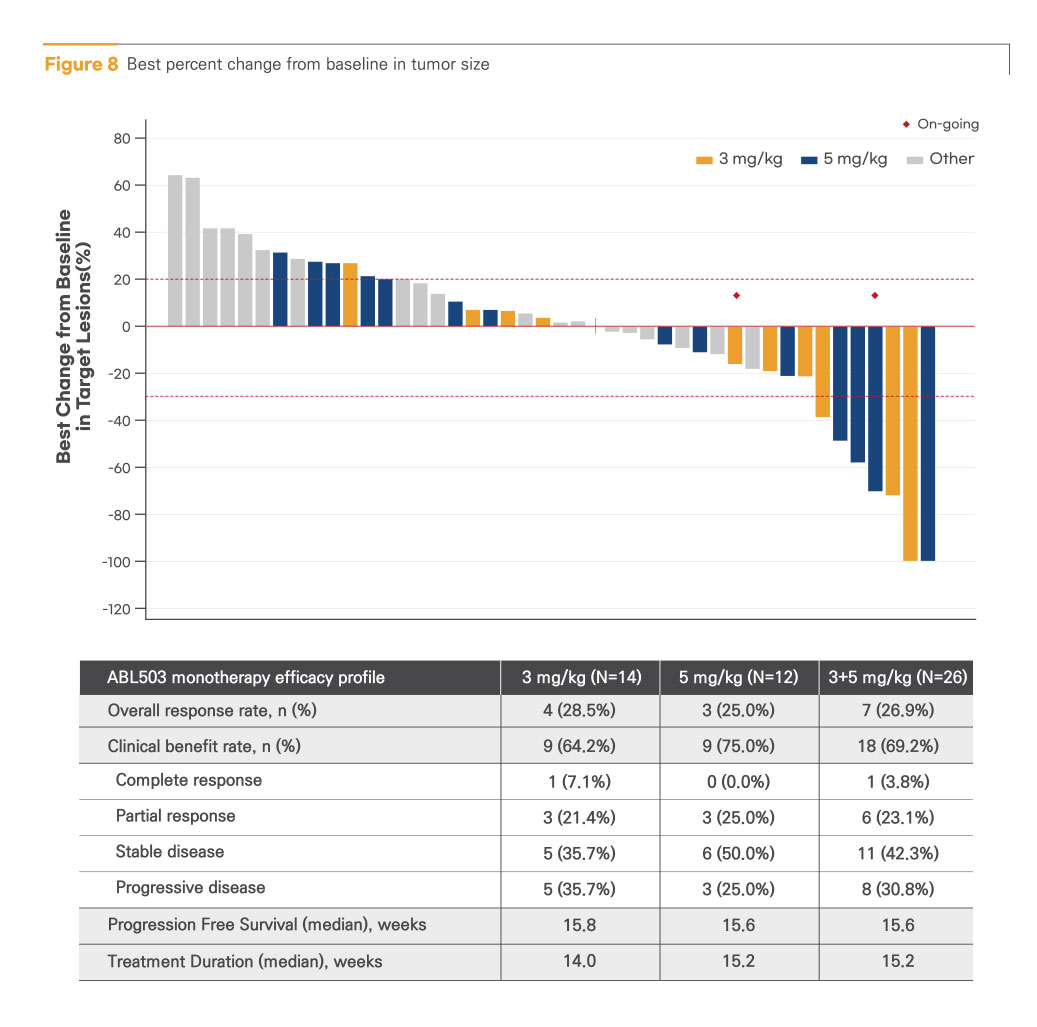
Promising response in prior checkpoint inhibitor (CPI) refractory/relapsed patients
5 out of 7 responders had received prior anti-PD-(L)1 inhibitors and all were relapsed or refractory to anti-PD-(L)1 inhibitors
Dose-limiting toxicities (DLT-evaluable set)

| Tumor type | BoR | Response to prior CPI |
|---|---|---|
| Ovarian cancer | CR | CPI relapsed |
| Ovarian Cancer | PR | Naïve |
| Head and Neck | PR | CPI relapsed |
| Gastric | PR | Naïve |
| Melanoma | PR | CPI relapsed |
| Esophageal | PR | CPI relapsed |
| Hepatocellular | PR | CPI relapsed |
Induced effector memory T cells by ragistomig
A representative case is that one patient achieved sustained tumor regression over 6 months, even after treatment discontinuation. It might be linked to the observed activation and expansion of effector memory T cell induced by ragistomig (Duration of response: 39 weeks)
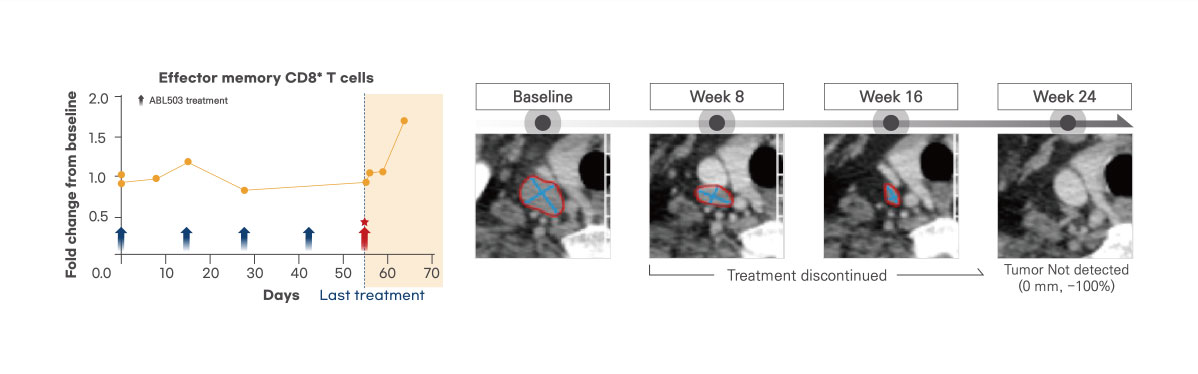
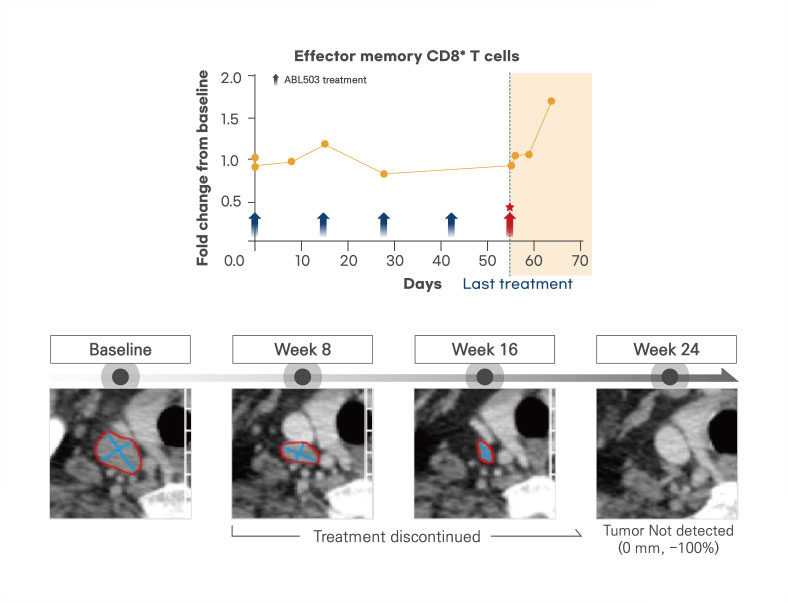
Tolerable safety profiles

| Preferred Term | All Grades | ≥ Grade 3 | ||
|---|---|---|---|---|
| n | % | n | % | |
| Any TRAE | 40 | 75.5 | 22 | 41.5 |
| ALT increased | 17 | 32.1 | 12 | 22.6 |
| AST increased | 16 | 30.2 | 11 | 20.8 |
| Nausea | 7 | 13.2 | - | - |
| Rash | 7 | 13.2 | 2 | 3.8 |
| Fatigue | 6 | 11.3 | 1 | 1.9 |
| Pyrexia | 8 | 15.1 | 1 | 1.9 |
| Platelet count decreased | 6 | 11.3 | 1 | 1.9 |
Some treatment related adverse event related to PD-L1 or 4-1BB targeting antibody occurred, but improved and manageable with corticosteroid once applied or ABL503 interruption
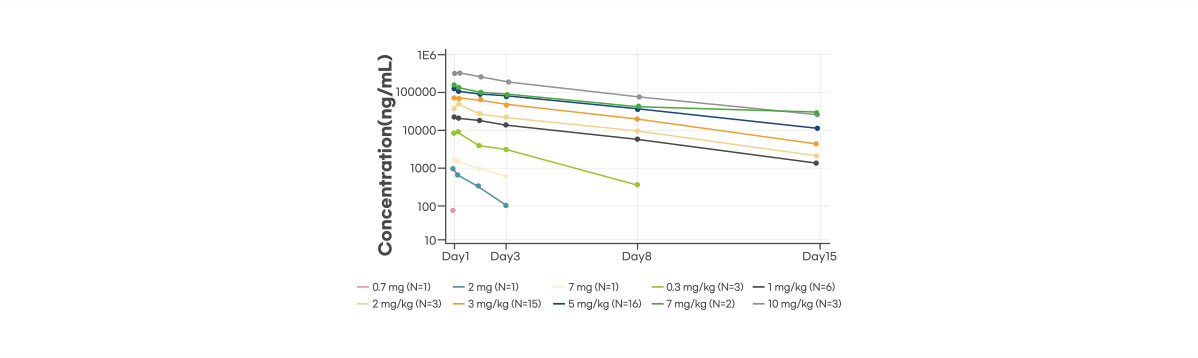
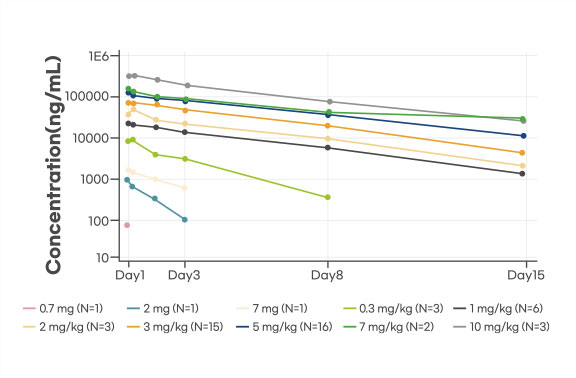
Pharmacokinetics(PK): Dose proportional PK was observed
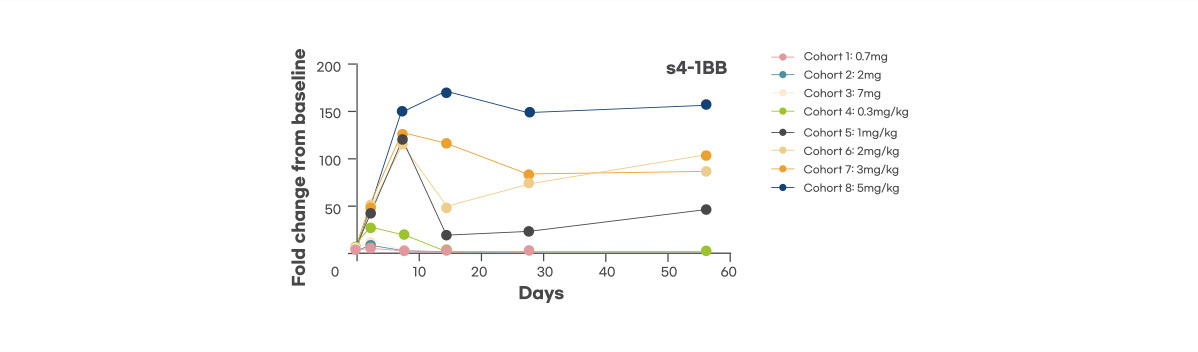
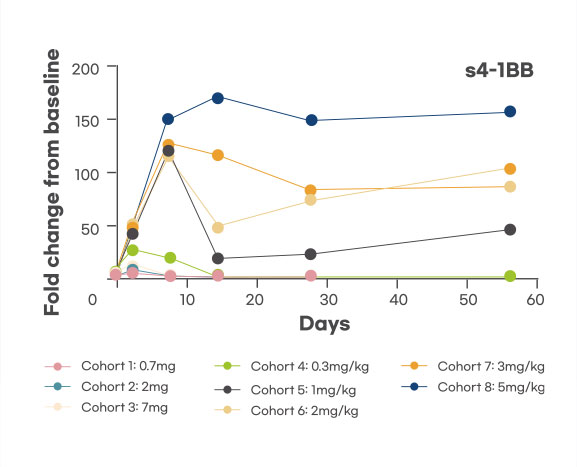
Pharmacodynamic biomarker
Dose-dependent increase of PD biomarker, s4-1BB was observed, demonstrating target engagement, and sustained at optimal dose level